FAQ
Tracking the latest COVID-19 developments
COVID-19 is among the most researched diseases in history and has generated public interest and conversation about the underlying science. A high volume of COVID-19 data from clinical trials, scientific journals, and drug research are emerging at a very rapid pace. At Private Health, we trust the science, we acknowledge what is known and not known yet, we check the facts for ourselves, we evaluate and explain the risks, and we recommend practical steps to mitigate them. We remain vigilant for new developments, but optimistic that COVID-19 is moving on the path from pandemic to endemic. Here is what you need to know right now.
Big milestone: 100 million shots in arms
The Biden Administration has reached its goal of 100 million shots in arms within the first 58 days in office. As of March 18th, 65% of people aged 65 or older have received at least one shot, and 36% are fully vaccinated. An average of two and a half million shots are being given each day, outpacing the rest of the world. We now have three very effective vaccines approved under the FDA Emergency Use Authorization (EUA) that will help us in the fight against COVID-19: Pfizer/BioNTech, Moderna, and Janssen (Johnson & Johnson (J&J)). Two additional companies, Novavax and AstraZeneca/Oxford, are expected to submit their vaccines for EUA approval soon. Amid the current intense public scrutiny of the AstraZeneca/Oxford vaccine, Private Health continues closely monitoring the landscape for all COVID-19-related clinical trial data, vaccine applications, approvals, and current literature reporting.
“65% of people aged 65 or older have received at least one shot, and 36% are fully vaccinated.”
President Biden
Here is Private Health’s current perspective on some key questions:
How successful is the federal government’s vaccination program? Are we making meaningful progress?
The federal government has expedited the production of critical materials such as supplies and equipment. Vaccine manufacturers have sped up the delivery of tens of millions of more doses of vaccines and are on track to have enough vaccine supply to inoculate every American by the end of May, months earlier than expected. With distribution sites expanding daily, it is anticipated that all adults will be eligible to be vaccinated no later than May 1st. At the current rate we could cross the threshold from pandemic to endemic by late summer/early fall.
Comparing the COVID-19 vaccines approved for the US
How do the Johnson & Johnson, Pfizer/BioNTech and Moderna vaccines differ?
There are a few key differences among the Pfizer/BioNTech (Pfizer), Moderna, and J&J vaccines, including the technologies used in their development, their storage requirements, and the number of doses needed. The J&J vaccine is made using a relatively traditional approach called viral vector technology, requires only one shot, and can easily be stored and shipped. The Pfizer and Moderna vaccines use messenger RNA (mRNA) technology, require two shots given three to four weeks apart, and must be kept frozen or in ultracold conditions.
The J&J and Moderna vaccines have been cleared for use in people 18 years and older, while the Pfizer vaccine can be used in people 16 and older. All three companies have trials underway testing the safety and efficacy of the vaccines in adolescents 12 years old and up. Moderna has a recently announced a trial for children ages 6 months and older. Despite their differences, it is important to keep in mind that all three vaccines effectively train the immune system to respond to the coronavirus and provide robust protection against hospitalization or death from a COVID-19 infection.
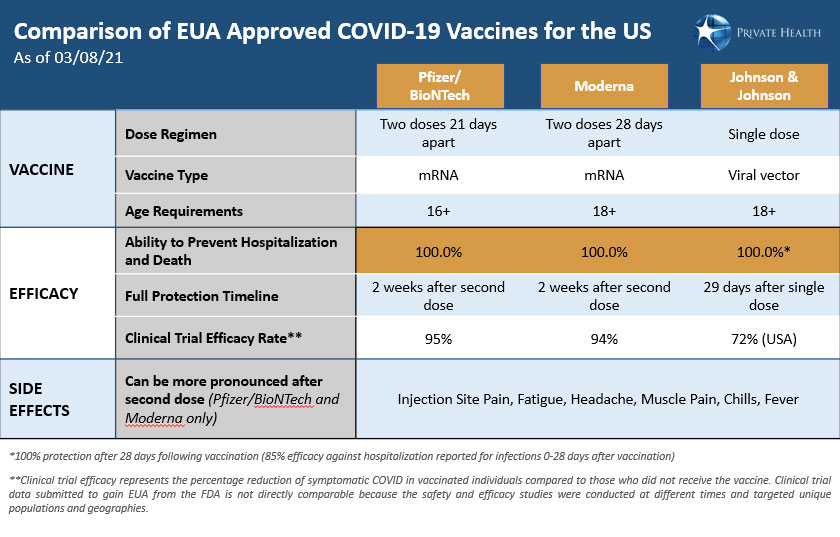
With Pfizer and Moderna able to achieve ~95% efficacy in preventing infection, will the J&J vaccine provide the needed protection against the coronavirus?
The J&J vaccine provides strong protection against COVID-19. Before any vaccine can be approved by the U.S. Food and Drug Administration (FDA) for emergency use, scientific data from clinical trials must be provided that demonstrate its efficacy. In the J&J trial, efficacy was reported as the chance that a person can avoid infection, serious illness, or death from COVID-19 once vaccinated as compared to a control group who received a placebo instead of the vaccine.
The J&J trial was conducted with ~40,000 people in eight countries across the globe during a time when the virus was surging. It also included regions where known variants were circulating, such as South Africa and Latin America. These factors all impact the reported efficacy rate and make it impossible to directly compare effectiveness between the J&J vaccine and the Pfizer and Moderna vaccines. That said, for US trial participants, the J&J vaccine was 72% effective at preventing moderate to severe COVID-19 infections; the figure for the global population tested was 66%. However, arguably the most important measure of the vaccine’s efficacy is how well it protects against hospitalization or death from COVID-19. The J&J vaccine was 85% effective in preventing severe disease across all regions and 100% effective against hospitalization or death from COVID-19 when assessed after 28 days following vaccination.
Why does the J&J vaccine only require one dose? Wouldn’t two doses make it more effective?
The J&J vaccine was demonstrated to be effective and received EUA based on results from a clinical trial testing a single dose of the vaccine. The single dose administration provides clear advantages in the ability to administer the vaccine more quickly and in hard-to-reach populations. J&J is currently running additional trials to determine if efficacy increases significantly when a second dose is given either one, two, or three months after the initial dose, as well as when a booster shot is given a year later.
What are the possible side effects of COVID-19 vaccines?
The reported side effects of COVID-19 vaccines are similar: mild to moderate and short-lasting, although the data suggests that side effects from the J&J vaccine may be generally less severe. Because the J&J vaccine is a single shot, you will only experience these side effects once. Side effects from vaccines are expected, typically occur within 48 hours of receiving a dose, and fully resolve after a few days. They are a good sign that your body is generating an immune response. The most commonly reported side effects include pain at the injection site, tiredness/fatigue, headache, muscle pain, chills, and joint pain. Although rare, allergic reactions have been reported for the Pfizer and Moderna vaccines; if you have a history of anaphylaxis or allergies to the ingredients within any vaccines, please consult your primary care physician before receiving a COVID-19 vaccine.
Can a vaccinated person still spread the coronavirus?
While vaccines are nearly 100% effective at preventing hospitalization or death from the virus, it is believed that vaccinated individuals can still spread COVID-19 or develop asymptomatic COVID-19. However, COVID-19 vaccines do not have to completely prevent infection to slow transmission. If the vaccines can limit the amount of virus in the body and reduce symptoms such as coughing and sneezing that facilitate the spread of the virus, the likelihood of transmission will be reduced as the number of vaccinated individuals increases.
What can a person do when they are fully vaccinated?
The Centers for Disease Control and Prevention (CDC) issued its first set of recommendations on activities that people who are fully vaccinated against COVID-19 can safely resume. This includes visiting with others who are fully vaccinated indoors and without wearing masks or staying six feet apart. This guidance also allows for visiting with unvaccinated people from other households, indoors and without wearing masks or staying six feet apart if everyone in the other household has a low risk for severe disease. It is important to remember that a person is considered fully vaccinated two weeks after receiving the second dose of Pfizer and Moderna and 29 days after receiving the single J&J shot. Private Health encourages everyone to take precautions when in public and when around unvaccinated people who are at high risk of getting severely ill from COVID-19.
The Latest on Variants
Will the variants in circulation today make the current vaccines less effective?
The CDC and other federal and global agencies are closely monitoring at least five variants that have been detected in the United States: B.1.1.7 (UK), B.1.351 (South Africa), P.1.(Japan/Brazil), B.1.427 (US-California) and B.1.429 (US-California). These variants have mutations in the virus genome that alter the characteristics and cause the virus to act differently in ways that are significant to public health. Additional studies will determine how much variants of concern impact the effectiveness of currently approved vaccines. Given the potential for increased contagiousness, the race to vaccinate in advance of another surge is critical. Variants are generated from replicating the virus inside the host. The better we control the rates of infection, the more we can reduce the impact variants will have and vaccination plays a big role.
How can someone protect themselves from the new variants?
We believe most people should get a government approved vaccine as soon as it is available to them. Until more people are vaccinated, it is also important to stay vigilant and continue to wear a mask (or double mask in high exposure risk scenarios by wearing a cloth mask over a surgical mask), maintain social distance, and wash your hands multiple times per day. If you are feeling unwell or know of an exposure to someone who may have COVID-19, get tested and contact your physician. We are close to controlling this virus, now is the time to remain vigilant; the near and long-term gains are worth it.
Therapeutic options for COVID-19 patients
Have treatments for patients with COVID-19 improved?
The scientific community has learned a lot about how to treat this illness at different points in the disease progression. We are gaining a better understanding of the disease and doctors are treating patients with earlier interventions and new medications including monoclonal antibodies and antiviral drugs with some success.
Far fewer people are being hospitalized and dying, and this trend parallels the overall infection trend, which has been dropping in the US since early January. In fact, hospitalization and death rates related to COVID-19 infections have been on a double-digit decline in 2021 and that downward trend is slightly faster than the decline in overall infection rate.
What types of treatments are being offered to patients?
Certain medications and supplements are being offered to COVID-19 positive patients, especially those at risk for more severe disease. Several supplements, over-the-counter medications, and prescription drugs may provide symptom relief, reduce the duration, and/or decrease the severity of a COVID-19 infection. COVID-19 positive patients convalescing at home can consider these options; the most appropriate regimen depends on clinical history, comorbidities, and risk level, and should be determined under the guidance of a clinician/physician. There are a number of clinical trials in progress to evaluate the efficacy of novel and existing drugs for the treatment of COVID-19. The currently EUA approved treatments which have received FDA review include remdesivir (an antiviral agent), convalescent plasma, and the monoclonal antibody treatments from Eli Lilly and Regeneron. These interventions are especially promising at mitigating severe COVID-19 symptoms. Many more clinical trials are underway.
Testing Matters
Does testing still matter?
Because we are currently working primarily to control the spread of COVID-19, we anticipate a continued need for testing as the disease enters a more endemic phase. Testing will continue to be complementary to vaccination through 2021 and likely beyond. It will remain an important component of a broader strategy to monitor the spread and prevalence of COVID-19 globally, as well as to protect vulnerable populations. Screening in public settings such as at schools, airports, sports arenas, entertainment venues, senior housing, and nursing homes will continue so that people feel safe returning to activities.
Workforce testing and monitoring will be important to ensure that employees can return to work safely. Company requirements will differ depending on the localized risk of exposure and the extent to which employees can follow safety work protocols, work remotely, and maintain protective “bubbles”.
As more people travel, airlines, states, and countries are requiring proof of being COVID-19 negative prior to travel and upon arriving at a new location. Before travelling, be sure to check regional guidelines for testing, quarantine, and health department reporting of travel.
Do fully vaccinated people need to be tested for COVID-19?
According to the CDC, the risk that fully vaccinated people could become infected with COVID-19 is low. According to this general guidance, fully vaccinated people with no COVID-like symptoms do not need to quarantine or be tested following an exposure to someone with suspected or confirmed COVID-19, as their risk of infection is low. However, fully vaccinated people who do not quarantine should still monitor for symptoms of COVID-19 for 14 days following an exposure. More risk averse organizations will likely still offer, or even require, some level of testing to increase the chance of identifying any infection early, before it has a chance to spread.
Any fully vaccinated people who experience symptoms consistent with COVID-19 should isolate themselves from others, be clinically evaluated for COVID-19, and tested for possible infection.
