Ask Our Experts
The latest COVID-19 intelligence
At Private Health Management, our team of PhD-led research experts continuously monitor the latest news, research, and developments for new insights on the COVID-19 pandemic to improve the health and safety of our clients. Here’s the latest FAQ:
With the recent escalating COVID-19 cases in Europe, can we expect another COVID-19 wave in the United States?
The pandemic has been nothing if not unpredictable, so we don’t know for certain how the increasing cases in Asia and Europe might play out in the US. But this recent rise in cases is an important reason to stay vigilant. As the pandemic continues to evolve, there are multiple factors that contribute to individual and community risk:
- Increased infectiousness: The BA.2 Omicron subvariant is the most infectious variant we have seen to date, and is making up a higher proportion of new cases (~86% globally and ~35% in the US as of March 19, 2022), extending the Omicron surge around the world.
- Reduced risk mitigation measures: Many countries and US states are lifting COVID-19 restrictions, including eliminating mask mandates, indoor capacity limits, proof-of-vaccination requirements, and testing requirements.
- Suboptimal vaccination levels: Only 65.5% of Americans have completed their initial vaccination series, and of those, only 44.8% have received their booster shot.
- Waning immunity: Regardless of whether you have been vaccinated, infected, or both, immunity wanes over time.
We have the means to protect ourselves by being mindful of the case and hospitalization rates in our state and local communities and adjusting our behavior accordingly, including getting vaccinated and/or boosted when eligible, wearing a mask in crowded indoor spaces as appropriate, and staying home and testing for COVID-19 when not feeling well.
How do I keep my immunity up to date?
Immunity against COVID-19 can come from vaccination and/or recent confirmed infection. A person’s immunity is up to date in any of the following scenarios:
- Second shot of mRNA vaccine received within the last five months
- First shot of Johnson & Johnson received within the last two months
- Booster shot received
- Infected with the Omicron variant within the past 90 days (Omicron became dominant in the U.S. in late December 2021)
The CDC recommends that those who have been vaccinated stay up to date on their COVID-19 vaccines, meaning that individuals should continue to receive all recommended COVID-19 vaccines, including any booster doses, whenever eligible.
On March 29, 2022, the FDA authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccine for individuals 50 years of age and older and certain immunocompromised individuals. Shortly after, the CDC released updated guidance supporting the FDA’s authorization. The second booster should be administered at least 4 months after the first booster dose of any approved COVID-19 vaccine. This authorization is based on emerging evidence of continued safety and improved protection against severe COVID-19, especially in those over 65 years of age and those over 50 years of age with underlying medical conditions.
Should I get a vaccine or booster if had COVID-19?
Studies have shown that getting vaccinated after infection or vice-versa provides stronger immunity than either vaccination or infection alone.1,2 If you had COVID-19, you should still get vaccinated. If you are currently positive for COVID-19, you should postpone getting vaccinated at least until your symptoms have resolved and you meet the criteria for discontinuing isolation, but not longer than 90 days post infection (when your immunity from infection may begin to wane). The appropriate timing will vary based on several factors, including the severity of symptoms, treatments received, and local and employer vaccine policies.
Has COVID-19 now become a treatable disease?
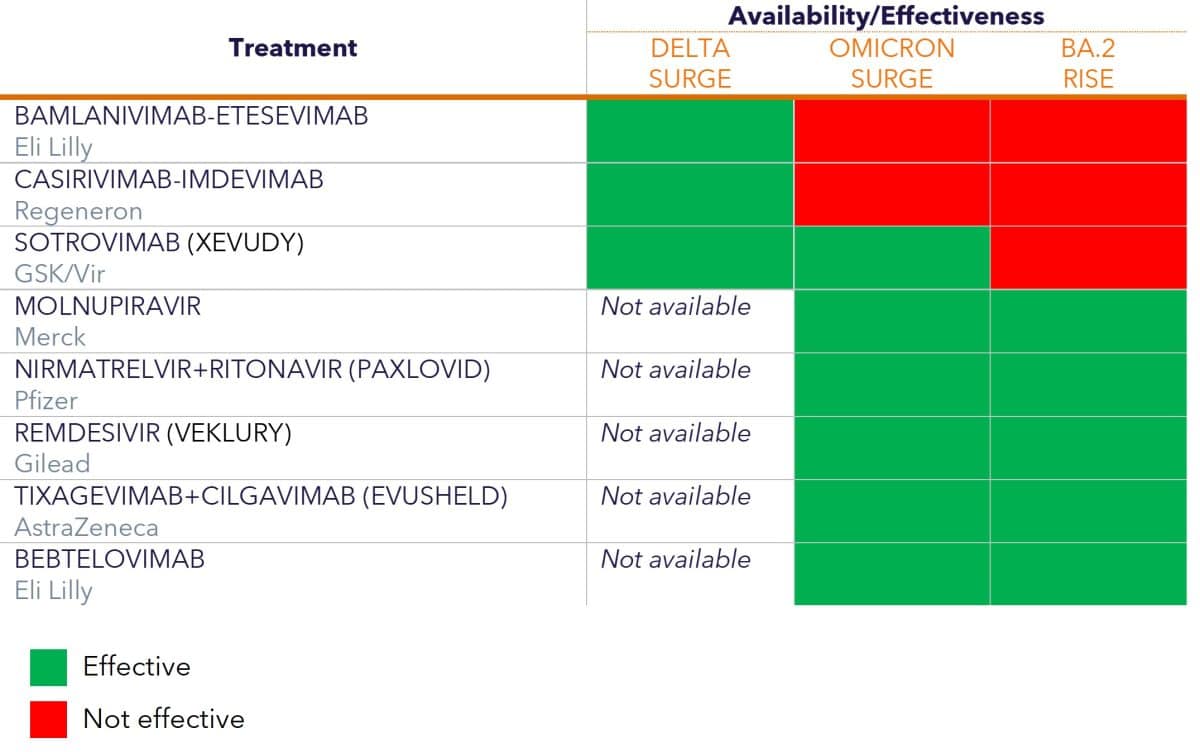
There are now several treatments available for individuals with mild/moderate COVID-19 who are at high risk for severe disease. However, as the virus evolves, treatments that were effective against one variant may not be as effective against the next variant (see Table 1 to see how different treatments have fared against the variants so far). Decisions regarding which treatment to use must take several factors into account:
- Effectiveness: The effectiveness of various therapeutics can vary; a treatment can be very effective against one variant, partially effective against another variant, and ineffective against a third variant. For example, the monoclonal antibody sotrovimab is effective against BA.1, but has reduced activity against BA.2; as a result, on March 25, 2022, the FDA updated the authorization of sotrovimab, removing its use in regions where BA.2 is responsible for more than 50% of cases.
- Dosing: In some instances, increased dosing can restore treatment effectiveness. For example, the FDA revised dosing for tixagevimab+cilgavimab (Evusheld) due to the rise of the Omicron variant.
- Variant ambiguity: When multiple variants or sublineages co-exist, as do BA.1 and BA.2 currently, it may not be feasible to know which variant is causing any particular infection. A treatment decision has to be made despite this information scarcity, emphasizing the importance of being aware of the dominant variants circulating in your region and which treatments are effective against them (Table 1).
- Administration: Some COVID-19 treatments are available in pill form, while others must be administered intravenously (IV). You should talk to your physician about which treatment might be best for you.
- Availability: Some treatments are currently more difficult to access than others, as they may not be widely available in clinics or pharmacies. The availability of treatments in any given area can be found using the COVID-19 Therapeutics Locator from the Office of the Assistant Secretary for Preparedness & Response.
During the current period where the Omicron variant is dominant, there are three antiviral drugs and two monoclonal antibody treatments that are available for people with mild to moderate COVID-19 who are at risk of progressing to severe disease. In addition, there is a monoclonal antibody treatment available for people who are not currently infected and have not been exposed to COVID-19 but are at high risk of progressing to severe disease. Some previously authorized monoclonal antibody treatments are not effective against Omicron and are not currently recommended.3,4
Table 1. Treatment Availability and Effectiveness Against COVID-19 Variants (treatments are not listed in order of priority; for a list of COVID-19 drugs ranked in order of preference, see the NIH guidelines)
To mask or not to mask?
Many jurisdictions are removing indoor mask mandates. However, just because there is no mask mandate in place does not mean you can’t or shouldn’t wear one. It is now up to each of us to decide if and when to continue wearing a mask. When faced with this decision, you should consider your health risks, the health risks of those close to you, the current status of COVID-19 in your community, the circumstances of your interactions with others (indoors vs outdoors, density of people, nature of the gathering, etc.), and your vaccination and prior infection status.
When you do decide to wear a mask, make sure it is well-fitting and completely covers your nose and mouth. High quality masks such as KN95, KF94, and N95 masks offer the best protection. Cloth masks are less effective against Omicron than against prior variants.
How can we use testing as a risk mitigation tool at work and at home?
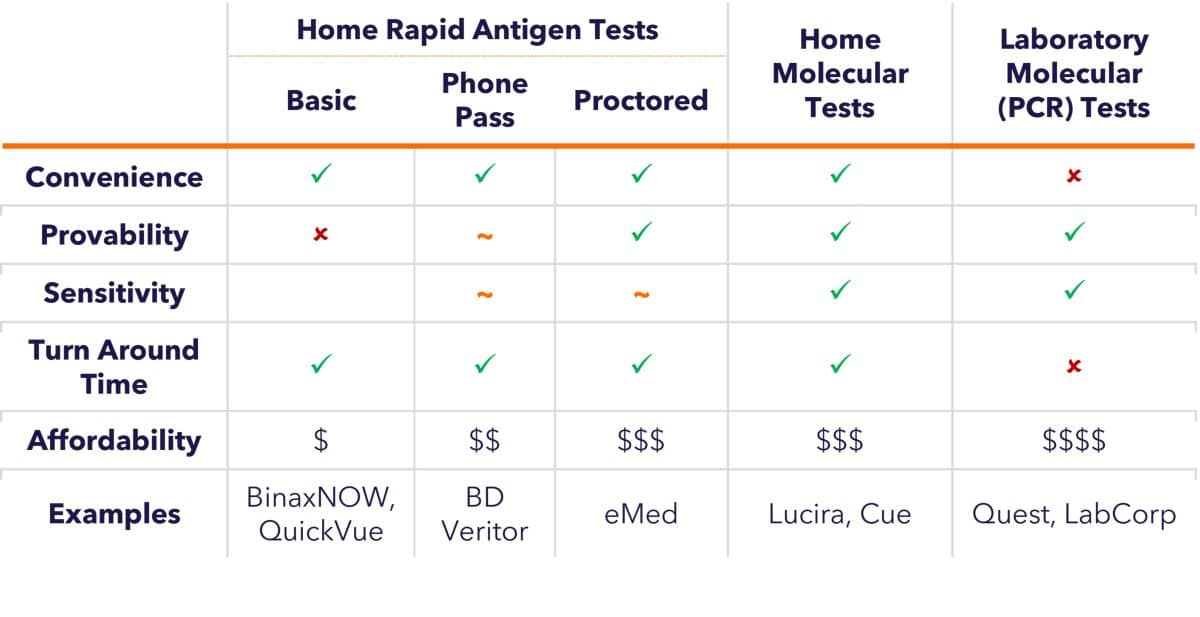
Testing remains an important tool for mitigating the risks associated with COVID-19 in our personal and professional lives, and in our communities. Just as the virus has evolved, COVID-19 test features have also evolved. Table 2 shows the different attributes of various tests to help guide test selection for any given situation. For instance: 1) you may enjoy the convenience and affordability of basic rapid antigen tests to determine whether you should isolate from friends and family; 2) certain venues may require proof of a negative test on your phone for entry, in which case the “Phone Pass” option is appealing; 3) proctored tests may be required for international travel; 4) if high sensitivity is critical, home or laboratory molecular tests are available.
Table 2. Features of COVID-19 Tests
When will the youngest children be protected?
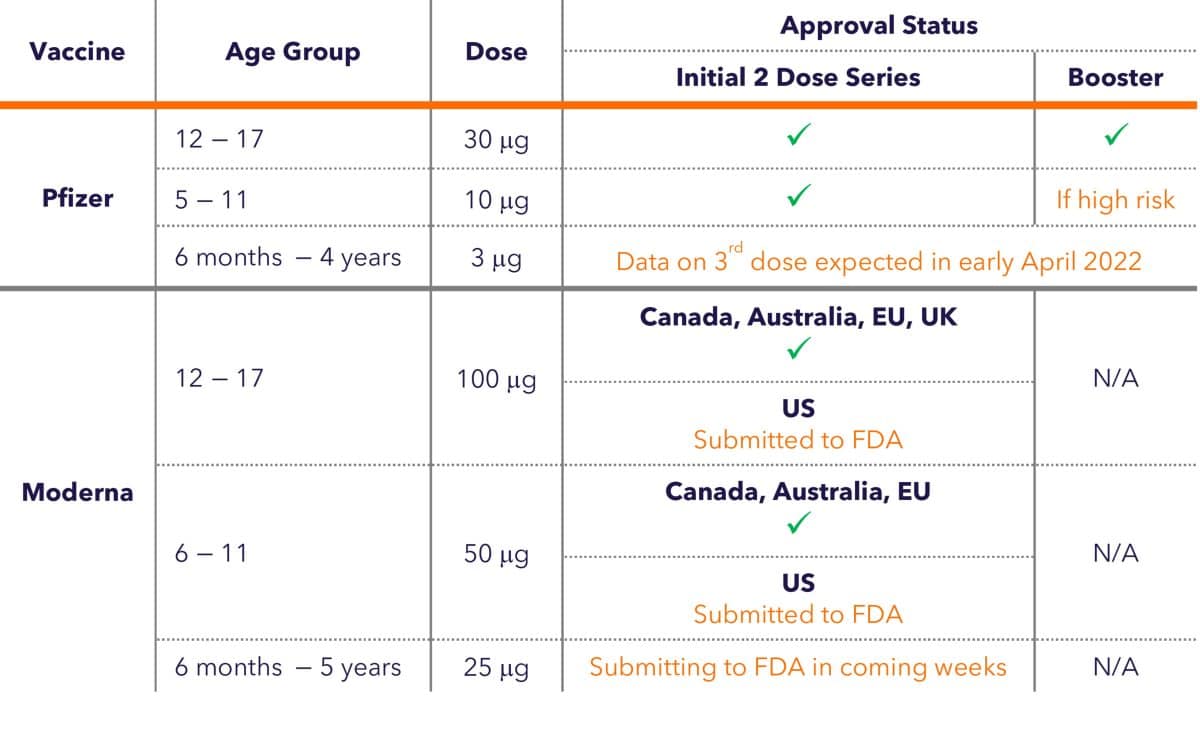
As shown in Table 3, all children 5 and above are eligible for vaccination, but vaccines for those under 5 are still under investigation.
Pfizer/BioNTech
In the US, the Pfizer-BioNTech vaccine is currently authorized for children 5-17 years of age; those 12 and over (and those between 5-11 who are at risk for severe disease) are also eligible for a booster shot. For those under 5, while the vaccine has been proven to be safe, the most effective dose and schedule are still under investigation. Data from a third shot is expected in early April 2022.
Moderna
In the European Union, Canada, and Australia, the Moderna vaccine is authorized for children 6-17 years of age; in the US, Moderna is currently authorized only for adults 18 and up. Moderna has initiated an Emergency Use Authorization (EUA) submission of its vaccine for children aged 6-11 and is updating its submission to the U.S. Food and Drug Administration (FDA) for authorization in adolescents aged 12-17. On March 23, 2022, Moderna announced positive interim data from their study of children 6 months to under 6 years of age and is planning on submitting a request for authorization to the FDA, the European Medicines Agency (EMA), and other global regulators in the coming weeks.
Table 3. Status of Vaccine Authorizations for Children
References
- Bates, T. A. et al. Antibody Response and Variant Cross-Neutralization After SARS-CoV-2 Breakthrough Infection. JAMA 327, 179-181, doi:10.1001/jama.2021.22898 (2022).
- Bates, T. A. et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Science Immunology 7, eabn8014, doi:doi:10.1126/sciimmunol.abn8014 (2022).
- Iketani, S. et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature, doi:10.1038/s41586-022-04594-4 (2022).
- Takashita, E. et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. New England Journal of Medicine, doi:10.1056/NEJMc2201933 (2022).